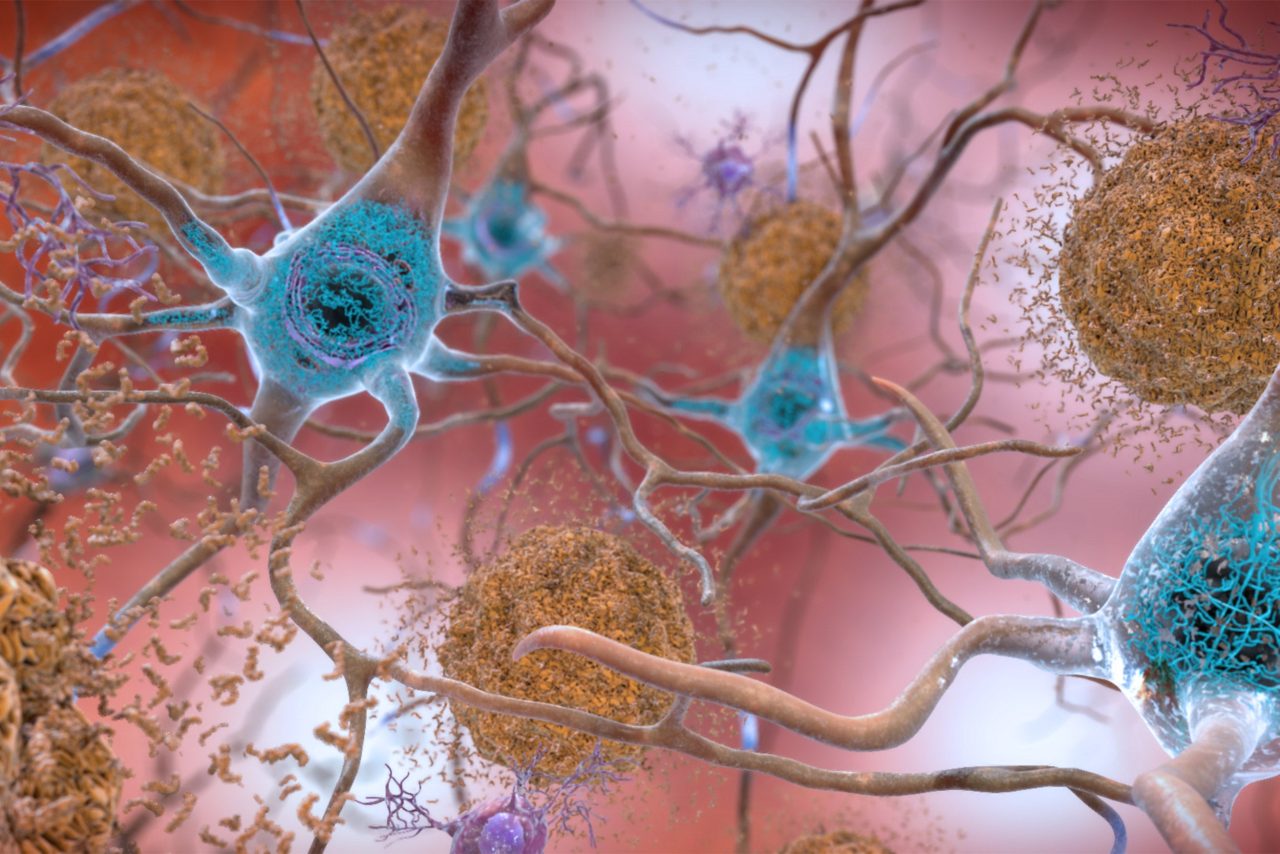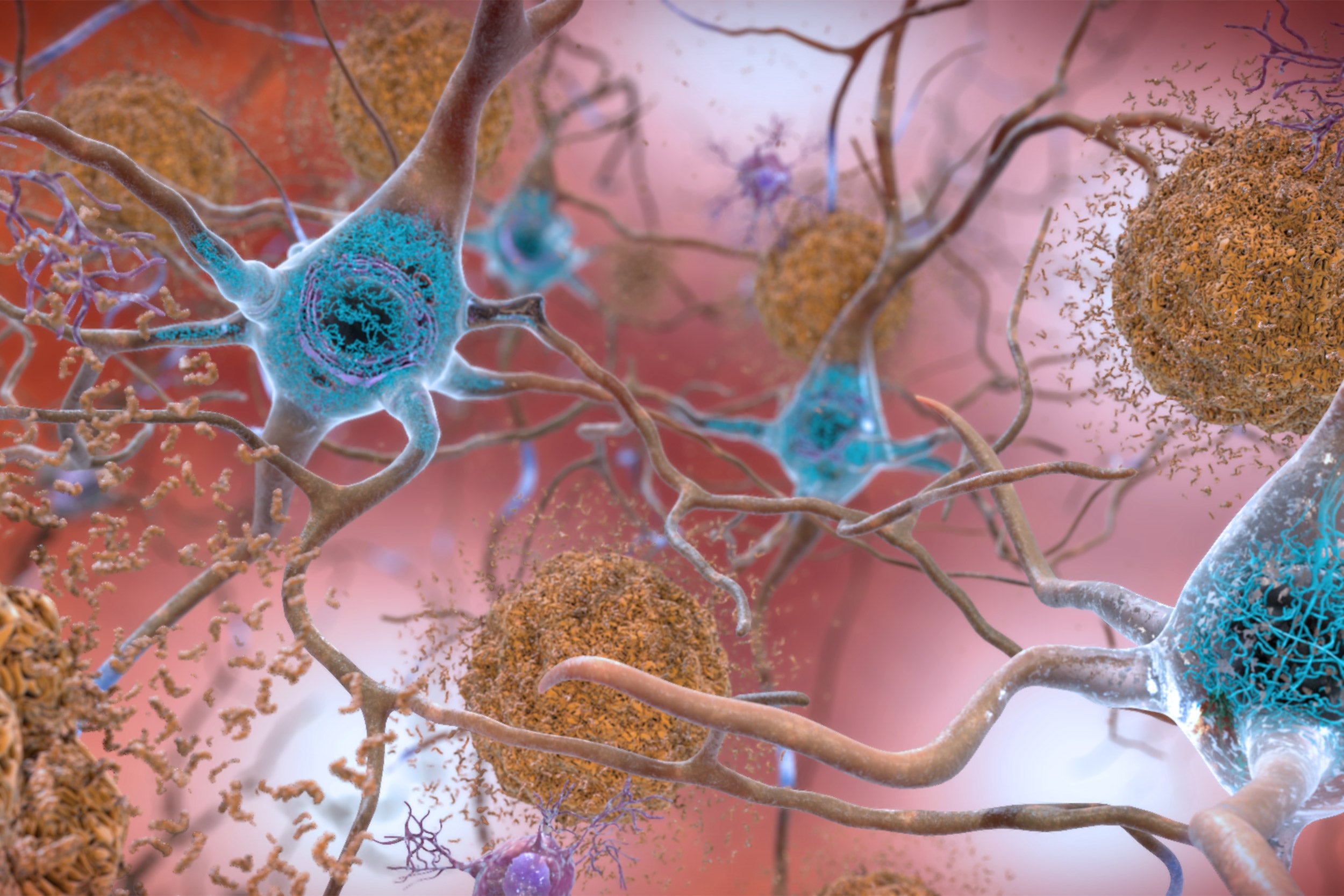
Beta-amyloid plaques and tau in the brain. Tau is a protein known to form pathological tangles in brains with Alzheimer’s disease. The patient studied had tau tangles in some regions of his brain, however minimal in the entorhinal cortex, whose functions include memory, navigation, and the perception of time.
National Institutes of Health
Health
Newly identified genetic variant protects against Alzheimer’s
Researchers find pathway for ‘resilience’ to dementia and which region of the brain to target with therapies
A new genetic variant that protects against Alzheimer’s disease has been identified by an international team led by Harvard Medical School investigators at Massachusetts General Hospital and Massachusetts Eye and Ear.
Their work, published May 11 in Nature Medicine, details the case of a patient with a genetic predisposition for developing early-onset Alzheimer’s disease. Despite the elevated risk for early onset, the patient remained cognitively intact until his late 60s.
The newly identified variant occurs in a different gene than the genetic variant from another individual in the same family, whose case was reported in 2019. But the new variant points to a common disease pathway.
Insights from the team’s findings also pinpoint a region of the brain that may provide an optimal treatment target in the future.
“The genetic variant we have identified points to a pathway that can produce extreme resilience and protection against Alzheimer’s disease symptoms,” said co-senior author Joseph Arboleda-Velasquez, associate professor of opthalmology at Mass Eye and Ear.
“These are the kinds of insights we cannot gain without patients. They are showing us what’s important when it comes to protection, and challenging many of the field’s assumptions about Alzheimer’s disease and its progression,” he said.”
The ‘Paisa’ mutation
The case that caught the investigators’ attention involved a family member of the world’s largest-known kindred with a genetic variant called the “Paisa” mutation (Presenilin-1 E280A).
Carriers of this variant usually develop mild cognitive impairment at a median age of 44, dementia at age 49, and die from complications of dementia in their 60s.
Francisco Lopera, director of the Neuroscience Group of Antioquia in Medellín, Colombia, a co-first author of the study, is the neurologist who identified this family and has been following them for the last 30 years.
The same team of investigators previously studied a woman from the same family who remained cognitively unimpaired until her 70s and whose case was reported in 2019.
In the new paper, the investigators described a case of a male carrier of the Paisa mutation who remained cognitively intact until age 67. He progressed to mild dementia at age 72 and died at 74 — decades after most people with the Paisa mutation typically do.
“The most exciting thing is that nature has revealed to us both the cause of Alzheimer’s and the cure for it.”
Francisco Lopera, director of the Neuroscience Group of Antioquia in Medellín, Colombia
“Extraordinary cases like this one illustrate how individuals and extended families with Alzheimer’s disease can help advance our understanding of the disease and open new avenues for discovery,” said co-senior author Yakeel Quiroz, a clinical neuropsychologist and neuroimaging researcher, an associate professor of psychology, and director of the Familial Dementia Neuroimaging Lab in the Departments of Psychiatry and Neurology at Massachusetts General Hospital.
“The insights we are gaining from this second case may guide us on where in the brain we need to look to delay and stop disease progression and will help us form new hypotheses about the series of steps that may actually lead to Alzheimer’s dementia,” Quiroz said.
“What we have done with the study of these two protected cases is to read Mother Nature,” said Lopera.
“The most exciting thing is that nature has revealed to us both the cause of Alzheimer’s and the cure for it. Mother Nature did an exceptional experiment with these two subjects: it endowed them both with a gene that causes Alzheimer’s and at the same time with another gene that protected them from the symptoms of the disease for more than two decades,” he said.
“Therefore, the solution is to imitate nature by developing therapies that mimic the mechanism of protection of these genetic variants in subjects at risk of suffering from the disease,” he added.
“A great door has been opened for the prevention and treatment of incurable diseases,” Lopera said.
The team’s efforts involved clinical assessments by investigators at the University of Antioquia in Colombia, genetic and molecular studies performed at Mass Eye and Ear, and Children’s Hospital Los Angeles, neuroimaging and biomarker studies conducted at Mass General, and neuropathological studies carried out by investigators at University Medical Center Hamburg-Eppendorf in Germany.
Biomarker study
The male patient was enrolled in the Mass General Colombia-Boston biomarker study (COLBOS), which brings members of an extended family group of 6,000 individuals with the known Paisa mutation to Boston for advanced neuroimaging, biomarker and genetic examinations.
The same study previously detected a case in which a female patient carried two copies of a rare Christchurch genetic variant, which affects APOE3 — a protein that is heavily implicated in Alzheimer’s disease. However, the researchers ruled out the presence of the APOE Christchurch genetic variant in the male patient.
The team performed genetic and molecular analyses at Mass Eye and Ear in collaboration with Xiaowu Gai and colleagues from Children’s Hospital Los Angeles to identify other variants that could have been protecting him from Alzheimer’s disease.
The most promising candidate was a new and rare variant, never before reported in the Reelin gene. The team named it Reelin-COLBOS.
In studies led by co-senior author Diego Sepulveda-Falla, a principal investigator at the Institute of Neuropathology at the University Medical Center Hamburg-Eppendorf, the team further verified the protective role of the Reelin-COLBOS variant in mouse models and neuropathological studies.
“Each of the protected cases, the APOE Christchurch and the Reelin-COLBOS case, shows a distinctive protective pattern in the postmortem analyses, one global and the other very localized,” said Sepulveda-Falla.
“These outstanding cases are teaching us that Alzheimer’s protection can take different shapes, and that perhaps a therapy can be successful just by targeting key brain structures such as the entorhinal cortex,” he said.
“They are forcing us to revise our previous concepts about neurodegeneration and cognitive decline. These are exciting times for us, and hopefully for the Alzheimer’s research field as well,” Sepulveda-Falla added.
The researchers described Reelin as a “cousin” of the more famous APOE protein. Both Reelin and APOE compete to bind to similar cellular receptors, essentially jostling to occupy the same seat.
When Reelin sits in the receptor seat, it diminishes the activation of tau, a protein known to form pathological tangles in brains with Alzheimer’s disease. When APOE binds the receptor, it has the opposite effect.
Reelin is a protein that plays a pivotal role in the regulation of brain cell development and function. In fact, previous reports have linked mutations in Reelin to diseases like autism, schizophrenia, epilepsy, and bipolar disorder.
Mutations linked to disease diminish the function of the protein, however, while in the case of Reelin-COLBOS, the protective variant increases the function of the protein.
“When we saw that one of our top candidates for the variant sat in Reelin, it was a bit shocking,” said Arboleda-Velasquez.
A key to understanding
“The fact that the first case showed us a variant affecting APOE and the second case affects Reelin tells us that this signaling pathway that controls the phosphorylation of tau, among other effects, may be key to understanding why these patients were protected,” he said.
“This is critical to guide therapies because it clearly tells us that more Reelin could potentially have beneficial effects.”
The most recent patient underwent neuroimaging exams at Massachusetts General Hospital at age 73. These scans revealed that while the patient’s amyloid-beta plaque burden was high, and he had tau tangles in some regions of his brain, another brain region called the entorhinal cortex had notably very minimal tau pathology.
ET imaging of RELN-COLBOS carrier showing limited aggregation of tau in the entorhinal cortex, an area of the brain that is characteristically affected in the early clinical stages of Alzheimer’s disease.
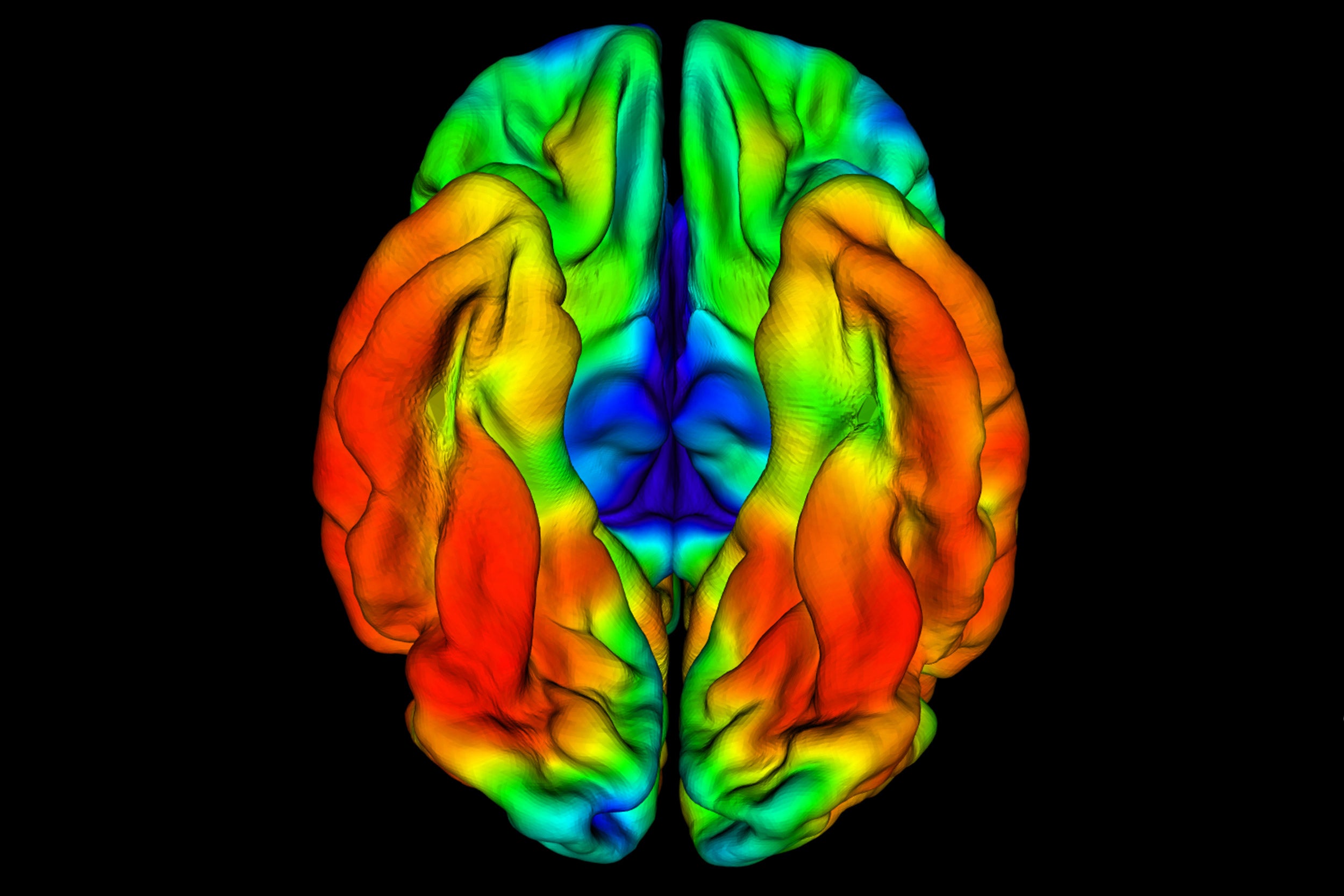
The entorhinal cortex plays a critical role in memory and learning, and its degeneration is known to lead to cognitive impairment and dementia. Studies in mice done for this paper also showed that the Reelin-COLBOS variant protected against tau pathology.
“This case indicates that the entorhinal region may represent a tiny target that’s critical for protection against dementia,” said Quiroz.
As investigators pursue the design of gene therapies to deliver treatments that modify or manipulate gene expression, understanding what brain region should be the precise target for delivery will become increasingly important.
Many treatments for Alzheimer’s disease, including drugs recently approved by the U.S. Food and Drug Administration, and other drugs currently in clinical trials, work to reduce amyloid plaque buildup.
New avenues for treatment
This study results point to new avenues for treatment because the two patients with nearly intact cognitive function had extremely high levels of amyloid in their brains, yet were protected.
“These exciting findings demonstrate the power of academic collaboration, where a retinal disease genetics expert working with a local neuroimaging authority can team up with leading neurologists and neuropathologists around the world to power scientific discovery,” said Joan Miller.
Miller is chair of ophthalmology at Mass Eye and Ear, Mass General Hospital, and Brigham and Women’s Hospital, and David Glendenning Cogan Professor and chair of ophthalmology at Harvard Medical School.
“Alzheimer’s disease remains a devastating disease with an immense global burden, and this work opens the door to further investigation into how this resilience pathway may lead to an effective therapeutic strategy,” Miller said.
The researchers noted that they cannot completely rule out that other factors, including additional gene variants, may have contributed to the patient’s resilience against Alzheimer’s disease symptoms.
More like this
But their experimental evidence in preclinical studies strongly implicate the Reelin-COLBOS variant.
Arboleda-Velasquez and Quiroz, together with Lopera and Sepulveda-Falla, plan to continue their work to identify additional protected patients from these Colombian families, learning from each extraordinary case.
They are also conducting research looking at treatments to target this protective pathway.
“It is a huge privilege to have these genetic cases to work on,” said Arboleda-Velasquez. “We are honored to be a part of the team that has made this discovery.”
Co-first authors of this publication are: Francisco Lopera, Claudia Marino, Anita S. Chandrahas, Michael O’Hare, and Nelson David Villalba-Moreno.
Disclosures: Arboleda-Velasquez and co-author Leo A. Kim are co-founders of Epoch Biotech, LLC, a biotech developing resilient case-inspired therapeutics. Quiroz and Lopera serve as consultants for Biogen.
This work was supported primarily through philanthropy to Mass Eye and Ear, including Open Philanthropy and Good Ventures, and the Remondi Family Foundation. The work was supported by National Institutes of Health (DP5 OD019833), National Institute on Aging (R01 AG054671), the Massachusetts General Hospital Executive Committee on Research (MGH Research Scholar Award), the Alzheimer’s Association, the National Institute of Neurological Disorders and Stroke (UH3 NS100121 and RF1 NS110048), the German Federal Ministry of Education and Research, UndoAD Project, the German Research Foundation (SFB877), and the API Colombia.