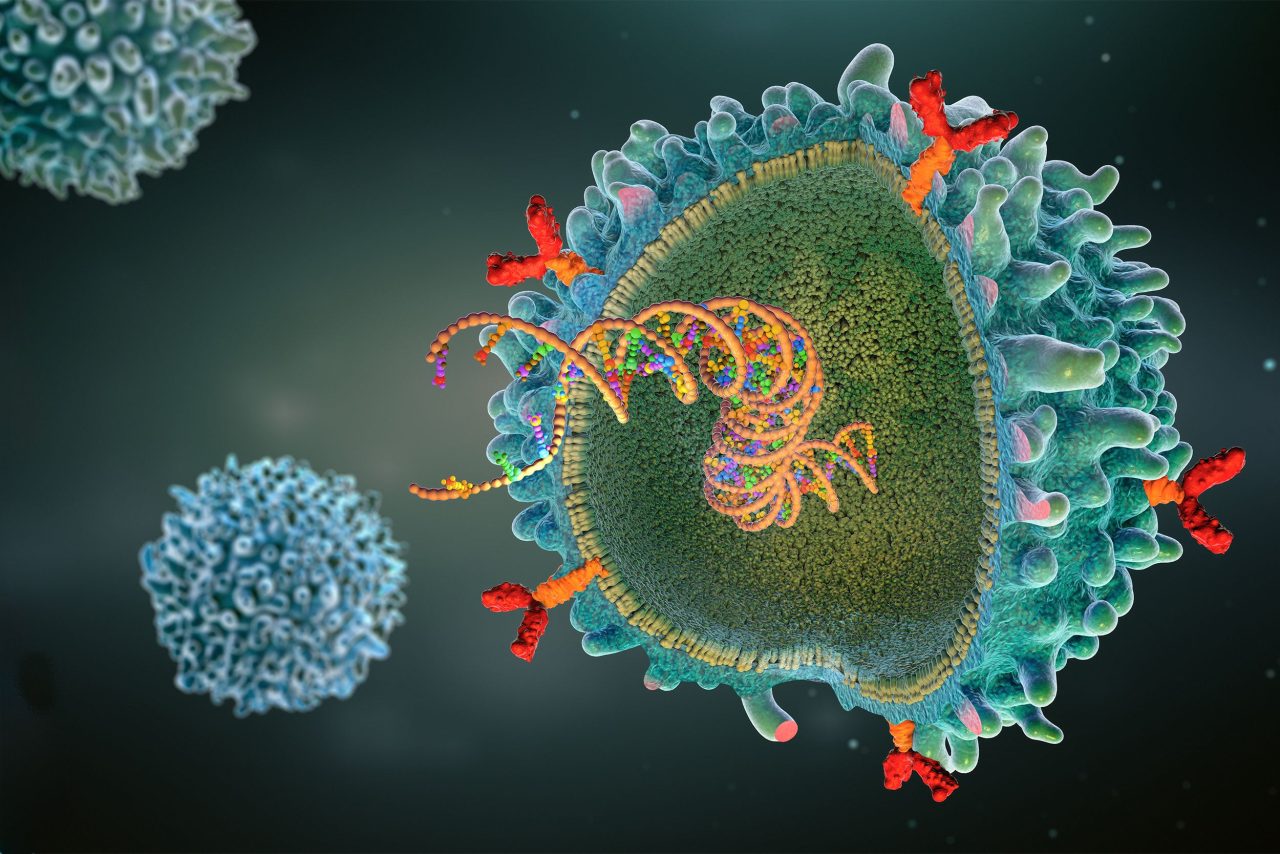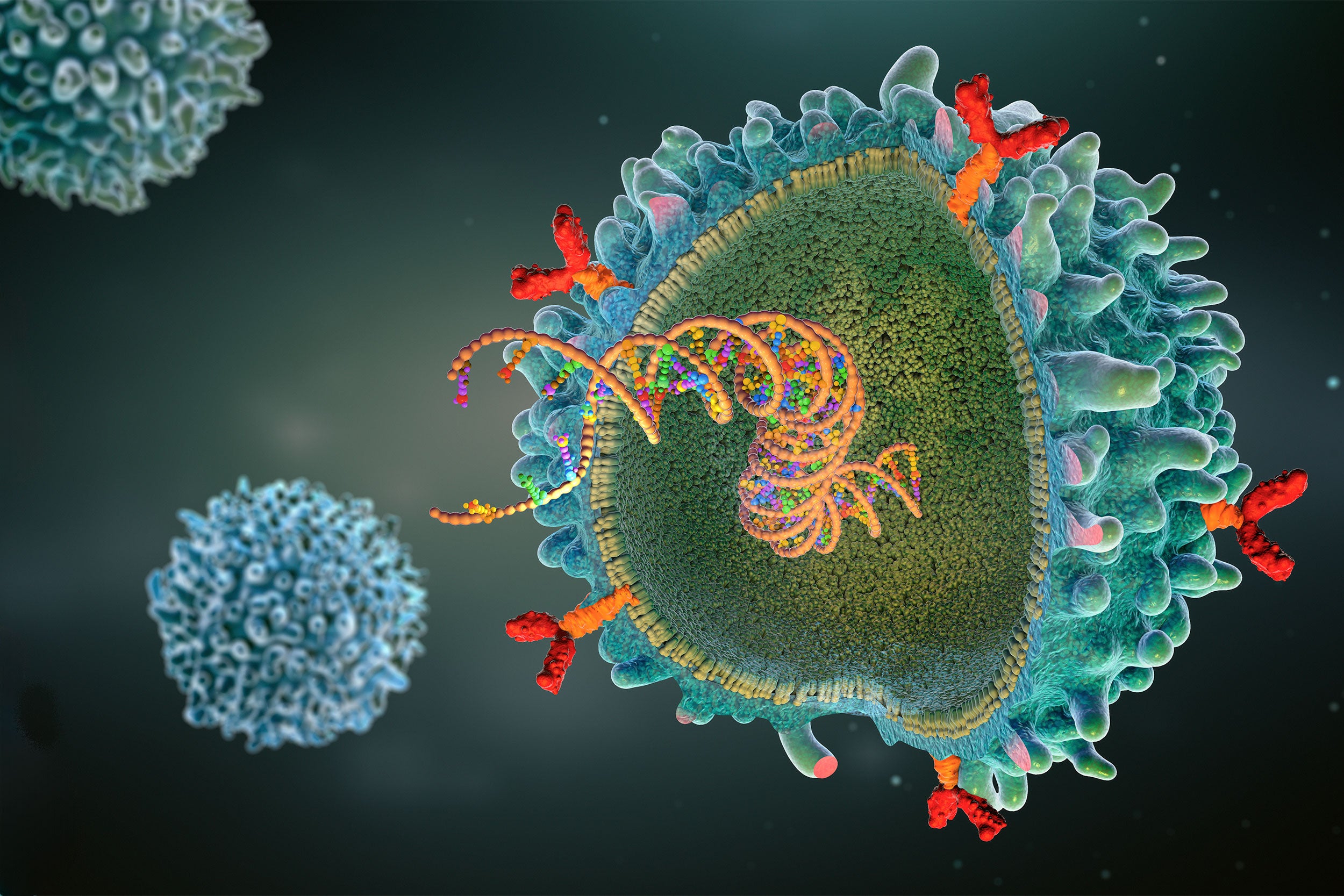
CAR (chimeric antigen receptor) T-cell illustration.
Christoph Burgstedt/Science Photo Library
Health
How serious is FDA warning about revolutionary blood-cancer treatment?
Dana-Farber researcher details promise, peril of CAR T-cell therapy, which enlists body’s immune system to fight disease
The Food and Drug Administration announced last week that it’s investigating reports of secondary cancers in patients who received CAR T-cell therapy, one of a suite of immunotherapies that have revolutionized cancer care over the past decade. The treatment reprograms a patient’s T cells, a key part of the immune system, to recognize and attack cancer cells.
So far CAR T-cell therapy has been used mainly for blood cancers, such as leukemia, lymphoma, and myeloma. Physicians have reported dramatic improvement in many patients who have exhausted more traditional treatments, like chemotherapy, radiation, and surgery.
The Gazette spoke with Eric Smith, an oncology researcher at the Dana-Farber Cancer Institute and assistant professor at Harvard Medical School, about the FDA’s announcement and what the implications might be for CAR T-cell therapy, a focus of Smith’s lab. This interview was edited for clarity and length.
Q&A
Eric Smith
GAZETTE: Can you give us a quick primer?
SMITH: CAR T-cell therapy has been around for over a decade. It’s a therapy where we engineer a patient’s own T cells with a virus that expresses a gene that we call a “chimeric antigen receptor,” or a CAR. That chimeric antigen receptor is encoded by a single synthetic gene comprised of fragments from a few different genes, including one for an antibody fragment that targets a protein on the surface of a cancer cell — CD19 for leukemias and lymphomas and BCMA for multiple myeloma.
The protein extends inside the T cell and, when that antibody fragment binds to the target antigen on the cancer cell, it causes the T cells to not only kill that cancer cell but also make more copies of itself, which reinforces the anticancer immunity.
There are now six FDA-approved versions of CAR T-cell therapy for different indications across leukemia, lymphoma, and myeloma.

GAZETTE: How have these therapies affected treatment of these cancers?
SMITH: These therapies have really been game-changing for the treatment of patients with blood cancers, who have relapsed, refractory disease. Quite often patients who have failed all other therapies get CAR T-cell therapy, and it causes dramatic and durable responses to their cancer.
On the CD19 side, up to 40 or 50 percent of patients can be cured from their blood cancer with this cell therapy. On the BCMA, myeloma side, more than 90 percent of patients respond to these therapies with remissions that can last a few years. The alternatives that these patients have are unlikely to approach anywhere near that sort of response rate. So it’s really transformed the field.
In addition, while our initial studies were done in patients in whom all other therapies have failed, more recently, randomized clinical trials are being done to see if we can provide these breakthrough cell therapies earlier in a patient’s disease course, increase the rate of cures, and prevent these patients from requiring additional lines of chemotherapy.
GAZETTE: What happened to these patients 10 years ago, before this treatment was on the scene?
SMITH: We really had no options for these patients, and they probably had a life expectancy of just a handful of months.
GAZETTE: The FDA last week said that they have reports of new cancer that seems to arise from the CAR T-cell treatment in 19 patients. Was it known that this was a potential issue?
SMITH: It was known, and, in fact, these therapies carried a warning that it’s a potential issue even before these reports came out.
The issue goes back to how the cells are made. We introduce a virus, usually a lentivirus, that will stably integrate the gene encoding the chimeric antigen receptor — the CAR — into the DNA of the T cells. That usually is tolerated very well and doesn’t lead to specific problems.
The theoretical risk is that the virus will integrate right in front of a gene associated with cancer, and the promoter — that gets the CAR to be made from the DNA inside the T cell — can increase production of the oncogene.
We’ve treated 35,000 patients — or thereabouts — with CAR T-cell therapies to date and really all the information that the FDA has revealed is that there were 19 cases out of 35,000. So, there’s a lot we still don’t know.
It’s unclear if that theoretical risk is actually what occurred. These are blood cancer patients who have received other therapies, are immunosuppressed, and are already predisposed to developing second blood cancers.
What we need to find out — and I’m sure the FDA, working with manufacturers, is looking into this — is if that theoretical risk, which up until now we had very rarely seen impact a patient, is in fact what’s happening in these patients. We need to know if and where the virus integrated in these cancer cells, which is something that can be determined.
And it can also be helpful to know how many copies of the virus are in those malignant cells. Oftentimes, you get one or two or three copies in an individual cell, but it could be more in some cells in some cases.
GAZETTE: You mentioned that other treatments can also cause cancer. That includes familiar treatments like chemotherapy and radiation. Do we know what the rates of secondary cancers from those treatments are and how it might compare to CAR T-cell therapy?
SMITH: That’s an excellent question but there are so many different chemotherapies, radiation therapies, and small molecules used to treat blood cancers, and every therapy is going to have a different incidence of secondary malignancies. So, it’s hard to answer that as a blanket question. Again, we need to know how many, if any, of those 19 cases are truly from the CAR T-cells.
GAZETTE: The FDA isn’t saying we should suspend using this therapy. Does that seem reasonable to you?
SMITH: That’s an excellent point. We talked about the benefits of this therapy, which is transformative for patients, with over 90 percent response rates for some indications. Even if all 19 of these patients are somehow directly linked to the CAR T-cell therapy, the benefits will still far outweigh the risks of developing a secondary malignancy.
GAZETTE: Can CAR T-cell therapy be used to treat this secondary cancer as well?
SMITH: There are CAR T-cell therapies in clinical trials targeting T-cell cancers. So it’s possible that these patients can get other treatments for this new T-cell lymphoma and that clinical trials of CAR T-cell therapies targeting those malignancies could play a role in the management of patients’ secondary cancer.
GAZETTE: Do we know how the 19 patients are doing and whether there have been any deaths among them?
SMITH: There were serious outcomes for some of these patients — I don’t know how many — and I do believe it included hospitalization and death, but the FDA has not revealed if that’s one death or more.
GAZETTE: In your lab, you work on these CAR T-cells and other cell-based therapies. Where do you see the future of this field heading?
SMITH: It’s been already a transformative field for oncology and for blood cancers specifically. With all the advances happening in synthetic biology and gene and cell engineering, we’re excited for a day when these therapies both reach more patients with different cancer types than the ones they’re already approved for and lead to cures in a higher percentage of patients.
It’s going to be exciting to send these therapies to solid tumors, to myeloid malignancies, and cut down on the relapse rate.
Another area that is exciting is that these therapies are made in clinical manufacturing labs. That takes resources and time, and delays getting patients the therapies as quickly as possible. In the future, one can imagine that we can drastically shorten manufacturing time or even go directly into patients with approaches that derive the CAR T-cells within the patient themselves.
GAZETTE: What advice would you give to people struggling with cancer who read coverage about this?
SMITH: First and foremost, talk with your oncologist if you have concerns. It’s a challenge even for physicians to understand everything that’s going on because there’s very little information available.
But the most important thing is to understand the benefits and the risks. With all cancer treatments, there are some risks. And in this particular case, given the low incidence of secondary malignancies and the lack of clarity around whether the CAR T-cell is directly responsible, I believe that the potential benefits of this therapy continue to far outweigh the risks.